Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kumagai, Yuta; Kimura, Atsushi*; Taguchi, Mitsumasa*; Watanabe, Masayuki
Radiation Physics and Chemistry, 191, p.109831_1 - 109831_8, 2022/02
Times Cited Count:0 Percentile:0.01(Chemistry, Physical)In this study, we investigated and compared the effects of a high-silica zeolite (HMOR) on the radiation-induced degradation of three aromatic chlorides, 2-chlorophenol (2-ClPh), 2-chloroaniline (2-ClAn), and 2-chlorobenzoic acid (2-ClBA), in order to examine its potential to reduce the influence of ions in water matrix in the irradiation treatment of water-soluble organic compounds. In the presence of ions reactive to radicals, the degradation of 2-ClPh in water was inhibited, but the combined use of HMOR much improved the degradation yield. This improvement was attributed to high performance of HMOR in adsorption of 2-ClPh. Similarly, HMOR was effective for adsorption of 2-ClAn and facilitated the 2-ClAn degradation by irradiation. In contrast, HMOR was poor at adsorption of 2-ClBA and consistently the degradation of 2-ClBA in the water-HMOR mixture was inhibited by the radical scavenger. These results demonstrate that HMOR can mitigate the influence of radical scavengers in water.
Hirade, Tetsuya
JPS Conference Proceedings (Internet), 25, p.011021_1 - 011021_2, 2019/03
Irradiation of water produce some reactive species such as OH radical. OH is formed by the reaction of a water cation and a water molecule just after ionization. On the other hand, a high energy positron injected in water will form cations and excess electrons even at the end part of the track. And hence, some positrons can form Positronium (Ps) with one of the excess electrons. The electrons in OH and Ps used to be in a same orbital in a water molecule before ionization of that water molecule. Therefore they were singlet at the time of the ionization. Every electron have each own hyperfine coupling constant after ionization. In water, reaction between Ps and OH, such as radical reaction or spin conversion, is possible. Therefore, quantum beats on these reaction can occur and the frequency of quantum beats will indicate the hyperfine coupling constant of OH which depends on the structure around OH. Therefore it is becoming possible to discuss the structure of water and reactivity of OH in the structure.
Hirade, Tetsuya
JPS Conference Proceedings (Internet), 25, p.011022_1 - 011022_3, 2019/03
OH radicals which are very reactive are formed by radiation decomposition in water. The behavior of OH radicals is important in corrosion of materials and reactions in living bodies. Recently, the reaction occurring between positronium (Ps) formed by OH radicals formed at the end of the positron track when positron is incident and positronium (Ps) formed by reaction of excess electrons formed with OH radical formation with the thermo-positron, it is reported that quantum beat occurs due to spin correlation. This quantum beat seems to have a period depending on the hyperfine coupling constant of OH radical. It is thought that the period and intensity of the quantum beat depends on the temperature, and it seems that it reflects the state around the OH radical. From the temperature dependence of the quantum beat detected by the reaction of this spin-correlated OH radical and triplet positronium we will explain what the liquid structure might be.
Yokota, Yuichiro; Funayama, Tomoo; Muto, Yasuko*; Ikeda, Hiroko; Kobayashi, Yasuhiko
International Journal of Radiation Biology, 91(5), p.383 - 388, 2015/05
Times Cited Count:10 Percentile:64.63(Biology)We investigated the dependence of the bystander cell-killing effect on radiation dose and quality, and related molecular mechanisms. Human fibroblasts were irradiated with  -rays or carbon ions and co-cultured with non-irradiated cells. Survival rates of non-irradiated cells decreased and nitrite concentrations in culture medium increased with increasing doses. Their dose responses were similar between
-rays or carbon ions and co-cultured with non-irradiated cells. Survival rates of non-irradiated cells decreased and nitrite concentrations in culture medium increased with increasing doses. Their dose responses were similar between  -rays and carbon ions. Treatment of the specific nitric oxide (NO) radical scavenger prevented reductions in survival rates of non-irradiated cells. Negative relationships were observed between survival rates and nitrite concentrations. From these results, it was concluded that the bystander cell-killing effect mediated by NO radicals in human fibroblasts depends on irradiation doses, but not on radiation quality. NO radical production appears to be an important determinant of
-rays and carbon ions. Treatment of the specific nitric oxide (NO) radical scavenger prevented reductions in survival rates of non-irradiated cells. Negative relationships were observed between survival rates and nitrite concentrations. From these results, it was concluded that the bystander cell-killing effect mediated by NO radicals in human fibroblasts depends on irradiation doses, but not on radiation quality. NO radical production appears to be an important determinant of  -ray- and carbon-ion-induced bystander effects.
-ray- and carbon-ion-induced bystander effects.
Yokota, Yuichiro; Funayama, Tomoo; Ikeda, Hiroko; Sakashita, Tetsuya; Suzuki, Michiyo; Kobayashi, Yasuhiko
JAEA-Review 2014-050, JAEA Takasaki Annual Report 2013, P. 75, 2015/03
We investigated the bystander effect induced by  -rays or carbon ions and analyzed the role of nitric oxide (NO) in the effect. Normal human fibroblasts were used. Cells inoculated on a porous membrane were irradiated with varying doses of
-rays or carbon ions and analyzed the role of nitric oxide (NO) in the effect. Normal human fibroblasts were used. Cells inoculated on a porous membrane were irradiated with varying doses of  -rays or carbon ions. Irradiated cells were then non-contact co-cultured with non-irradiated cells for 24 h. After co-culture, the survival rates of non-irradiated bystander cells co-cultured with irradiated cells decreased with increasing dose and bottomed out at 0.5 Gy or higher doses. This indicates that the bystander effect is dependent on irradiation dose but independent of radiation quality. Next, a specific NO scavenger c-PTIO was added to the culture medium during irradiation and co-culture. This treatment prevented the reduction in survival rates of bystander cells, clearly indicating that NO has an important role in the bystander effect.
-rays or carbon ions. Irradiated cells were then non-contact co-cultured with non-irradiated cells for 24 h. After co-culture, the survival rates of non-irradiated bystander cells co-cultured with irradiated cells decreased with increasing dose and bottomed out at 0.5 Gy or higher doses. This indicates that the bystander effect is dependent on irradiation dose but independent of radiation quality. Next, a specific NO scavenger c-PTIO was added to the culture medium during irradiation and co-culture. This treatment prevented the reduction in survival rates of bystander cells, clearly indicating that NO has an important role in the bystander effect.
Taguchi, Mitsumasa; Kojima, Takuji
Radiation Research, 163(4), p.455 - 461, 2005/04
Times Cited Count:25 Percentile:57.4(Biology)no abstracts in English
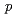 -nonylphenols in water by
-nonylphenols in water by  Co
Co  -ray irradiation
-ray irradiationKimura, Atsushi; Taguchi, Mitsumasa; Kojima, Takuji; Hiratsuka, Hiroshi*; Namba, Hideki
JAERI-Research 2004-018, 49 Pages, 2005/01
no abstracts in English
 " observation of guanine radicals induced by ultrasoft X-ray irradiation around the K-edge regions of nitrogen and oxygen
" observation of guanine radicals induced by ultrasoft X-ray irradiation around the K-edge regions of nitrogen and oxygenYokoya, Akinari; Akamatsu, Ken; Fujii, Kentaro; Ukai, Masatoshi*
International Journal of Radiation Biology, 80(11-12), p.833 - 839, 2004/12
Times Cited Count:8 Percentile:48.81(Biology)no abstracts in English
Taguchi, Mitsumasa; Kojima, Takuji
JAERI-Review 2004-025, TIARA Annual Report 2003, p.139 - 140, 2004/11
no abstracts in English
Taguchi, Mitsumasa; Kojima, Takuji
JAERI-Review 2004-025, TIARA Annual Report 2003, p.141 - 142, 2004/11
no abstracts in English
Taguchi, Mitsumasa; Kojima, Takuji
JAERI-Review 2003-033, TIARA Annual Report 2002, p.141 - 142, 2003/11
no abstracts in English
Yoshida, Yoichi*; Yang, J.*; Seki, Shuhei*; Saeki, Akinori*; Tagawa, Seiichi*; Shibata, Hiromi*; Taguchi, Mitsumasa; Kojima, Takuji; Namba, Hideki
JAERI-Review 2003-033, TIARA Annual Report 2002, p.145 - 146, 2003/11
no abstracts in English
Hirade, Tetsuya
Radiation Physics and Chemistry, 68(3-4), p.375 - 379, 2003/10
Times Cited Count:17 Percentile:72.74(Chemistry, Physical)The trapped electrons or anions in molecular solids and polymers cause the enhancement of positronium formation at low temperatures during positron annihilation lifetime (PAL) measurement. This method can be applied to investigate trapped electrons and anions in polymers. The advantage of the method is that the any kinds of trapped electrons or anions can be observed. We clarified that the glow peak appeared around 240K was caused by the trapped electrons on -CH- radicals by PAL experiment.
 -ray irradiation
-ray irradiationAbe, Yasuhiro*; Takigami, Machiko; Sugino, Koji*; Taguchi, Mitsumasa; Kojima, Takuji; Umemura, Tomonari*; Tsunoda, Kinichi*
Bulletin of the Chemical Society of Japan, 76(8), p.1681 - 1685, 2003/08
Times Cited Count:5 Percentile:26.49(Chemistry, Multidisciplinary)The decomposition of phenolic endocrine disrupting chemicals (P-EDCs), such as phenol, 4-butylphenol (BuP), and bisphenol A (BPA), in aqueous solutions by potassium permanganate (KMnO ) was studied and its efficiency was compared with that of hydroxyl radicals (OH
) was studied and its efficiency was compared with that of hydroxyl radicals (OH ) generated by
) generated by  Co
Co  -ray irradiation. Various organic acids and inorganic carbon were formed in the decomposition of P-EDCs due to either KMnO
-ray irradiation. Various organic acids and inorganic carbon were formed in the decomposition of P-EDCs due to either KMnO or OH
or OH . They were formed via direct aromatic ring cleavage in the case of KMnO
. They were formed via direct aromatic ring cleavage in the case of KMnO and OH
and OH addition-substitution reactions followed by aromatic ring cleavage in the case of OH
addition-substitution reactions followed by aromatic ring cleavage in the case of OH . Comparing the decrease in the P-EDCs based on the number of electrons, the amount of KMnO
. Comparing the decrease in the P-EDCs based on the number of electrons, the amount of KMnO spent to completely eliminate BuP and BPA was comparable to the amount of OH
spent to completely eliminate BuP and BPA was comparable to the amount of OH . Although three times more KMnO
. Although three times more KMnO was needed for phenol than OH
was needed for phenol than OH , the complete conversion of phenol into organic acids and inorganic carbon was achieved with 720
, the complete conversion of phenol into organic acids and inorganic carbon was achieved with 720 M of electrons in both cases.
M of electrons in both cases.
Koizumi, Hitoshi*; Ichikawa, Tsuneki*; Taguchi, Mitsumasa; Kobayashi, Yasuhiko; Namba, Hideki
Nuclear Instruments and Methods in Physics Research B, 206, p.1124 - 1127, 2003/05
Times Cited Count:8 Percentile:50.24(Instruments & Instrumentation)no abstracts in English
Kim, H.*; Hakoda, Teruyuki; Kojima, Takuji
Journal of Physics D; Applied Physics, 36(5), p.473 - 481, 2003/03
Times Cited Count:6 Percentile:28.18(Physics, Applied)no abstracts in English
 radical embedded in superfluid helium around 1.5 K
radical embedded in superfluid helium around 1.5 KWada, Akira; Aratono, Yasuyuki
Chemistry Letters, 32(2), p.200 - 201, 2003/02
Times Cited Count:6 Percentile:30.03(Chemistry, Multidisciplinary)no abstracts in English
Yokoya, Akinari; Akamatsu, Ken; Fujii, Kentaro
Nuclear Instruments and Methods in Physics Research B, 199, p.366 - 369, 2003/01
Times Cited Count:3 Percentile:27.69(Instruments & Instrumentation)no abstracts in English
Hirota, Koichi; Hakoda, Teruyuki; Arai, Hidehiko; Hashimoto, Shoji
Radiation Physics and Chemistry, 65(4-5), p.415 - 421, 2002/11
Times Cited Count:25 Percentile:81.52(Chemistry, Physical)no abstracts in English
Kojima, Takuji
Sangyo To Denki, (602), p.13 - 18, 2002/11
no abstracts in English